Cells, Free Full-Text
Por um escritor misterioso
Descrição
Long, noncoding RNAs (lncRNAs) are indispensable for normal cell physiology and, consequently, are tightly regulated in human cells. Yet, unlike mRNA, substantially less is known about the mechanisms for lncRNA degradation. It is important to delineate the regulatory control of lncRNA degradation, particularly for lncRNA telomeric repeat-containing RNA (TERRA), as the TERRA-telomere R-loops dictate cell cycle progression and genomic stability. We now report that the exosome complex component Exosc9 degrades lncRNA TERRA in human mammary epithelial cells. Heterochromatin protein 1 alpha (HP1α) recruits Exosc9 to the telomeres; specifically, the SUMO-modified form of HP1α supports interaction with Exosc9 and, as previously reported, lncRNA TERRA. The telomeric enrichment of Exosc9 is cell cycle-dependent and consistent with the loss of telomeric TERRA in the S/G2 phase. Elevated Exosc9 is frequently observed and drives the growth of endocrine therapy-resistant (ET-R) HR+ breast cancer (BCa) cells. Specifically, the knockdown of Exosc9 inversely impacts telomeric R-loops and the integrity of the chromosome ends of ET-R cells. Consistently, Exosc9 levels dictate DNA damage and the sensitivity of ET-R BCa cells to PARP inhibitors. In this regard, Exosc9 may serve as a promising biomarker for predicting the response to PARP inhibitors as a targeted monotherapy for ET-R HR+ BCa.
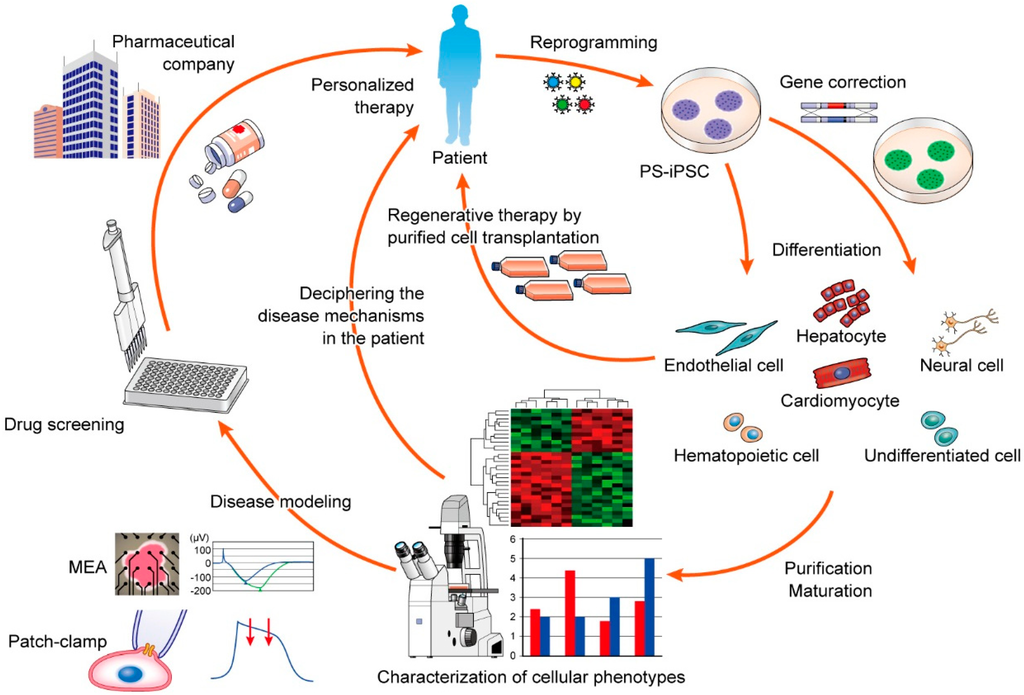
IJMS, Free Full-Text
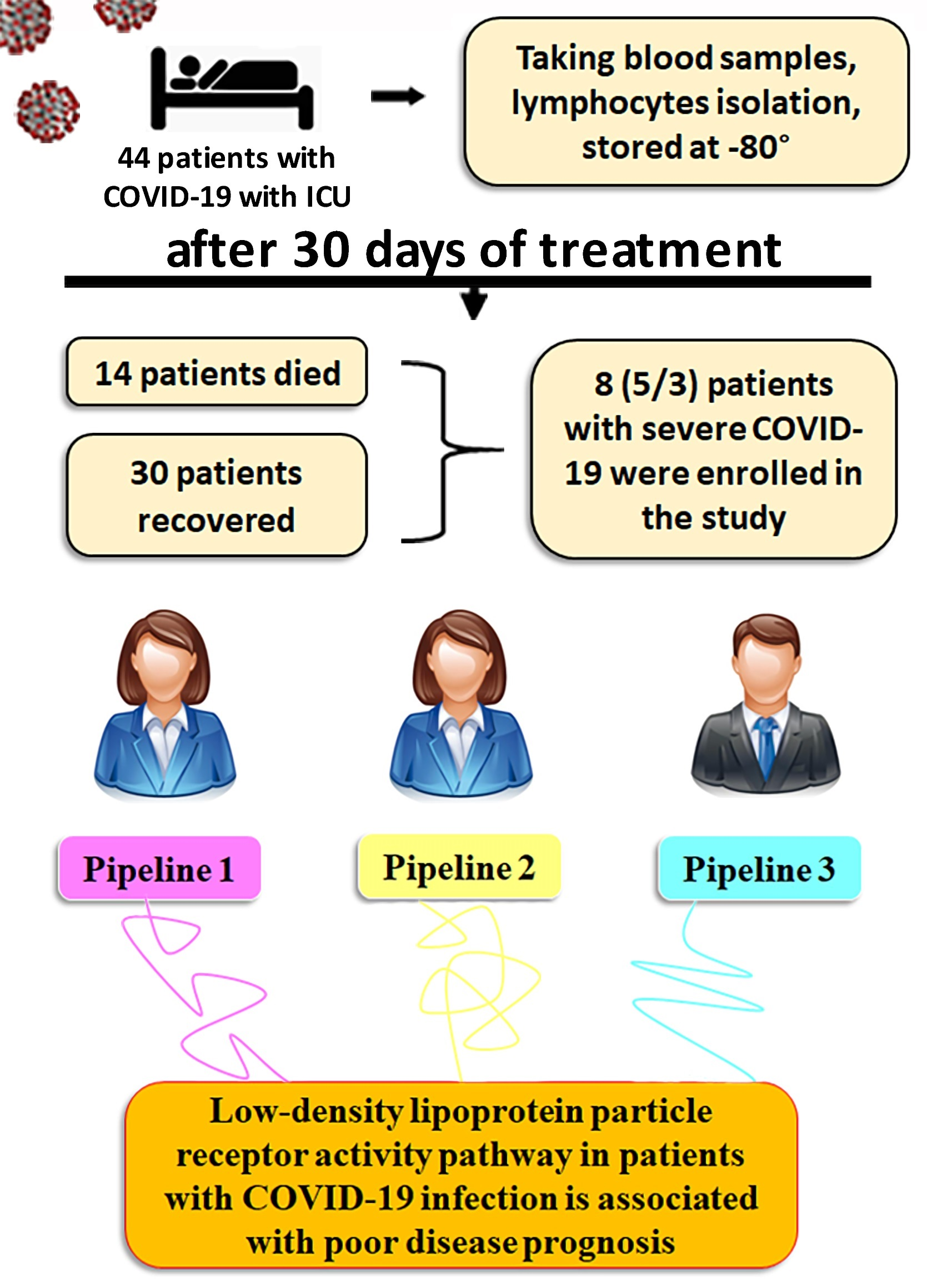
Cells, Free Full-Text
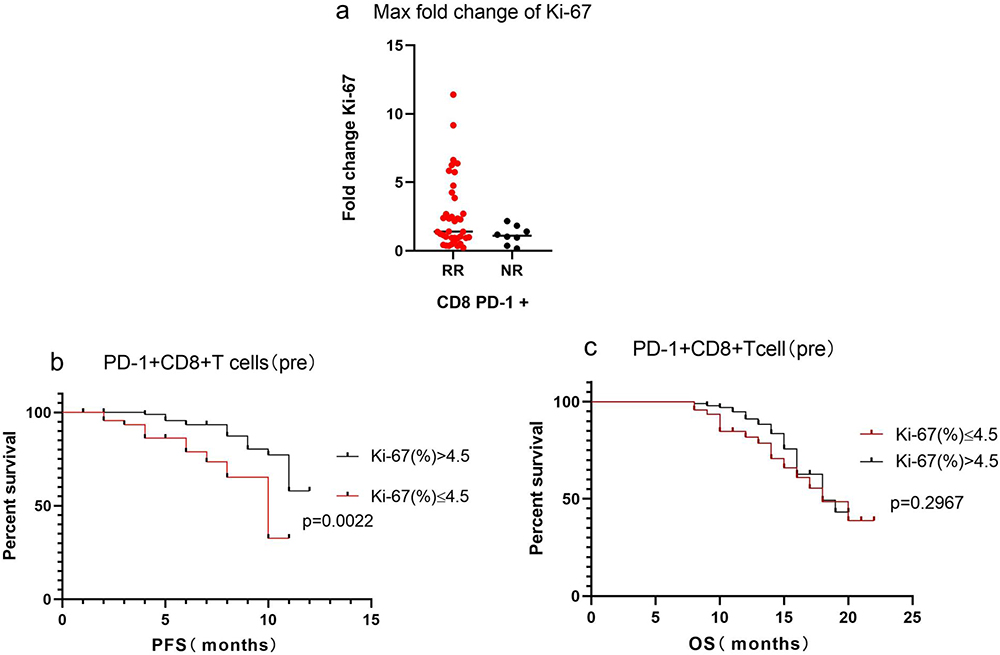
Cells, Free Full-Text
Full-spectrum cell-free RAN for 6G systems: s
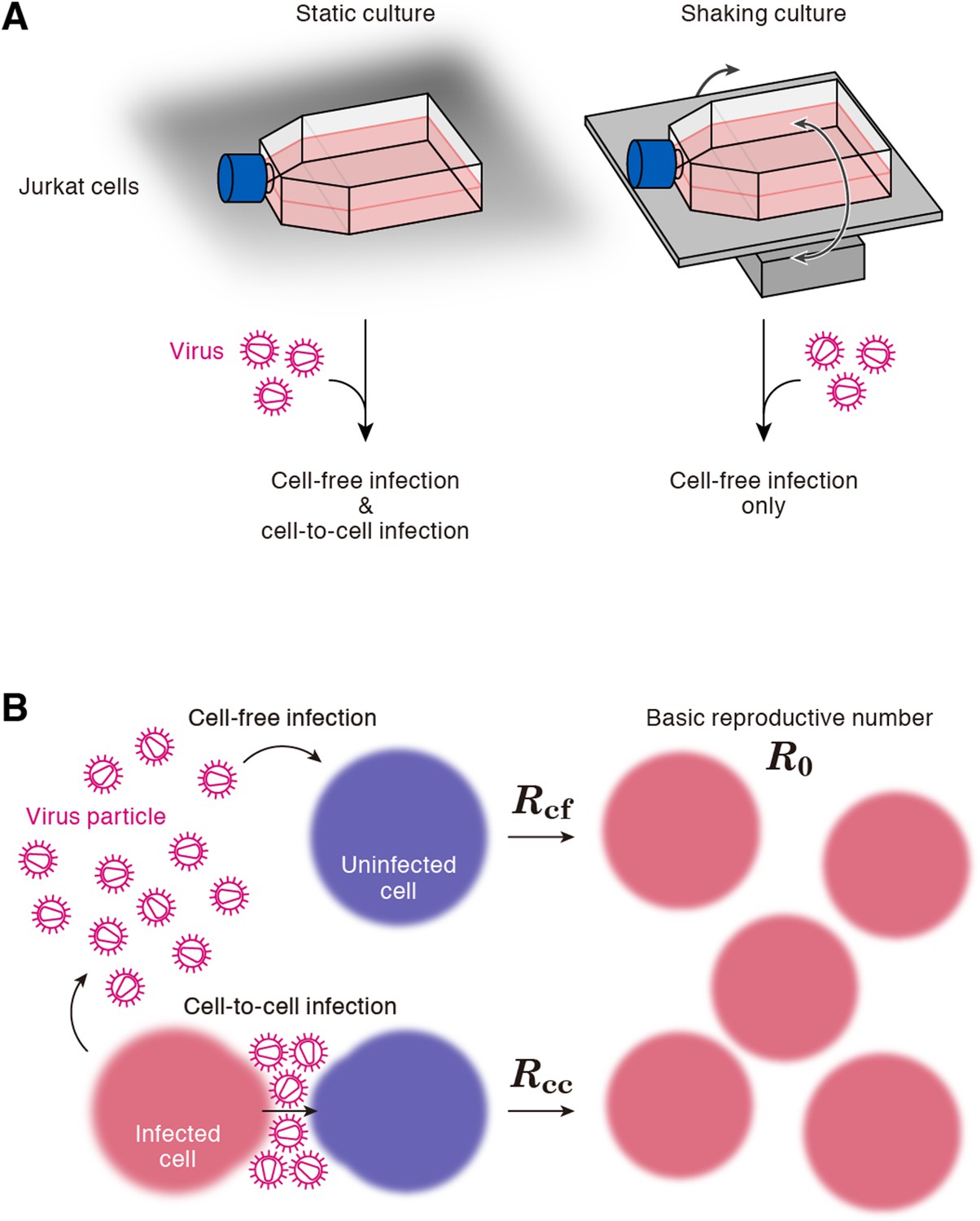
Cells, Free Full-Text
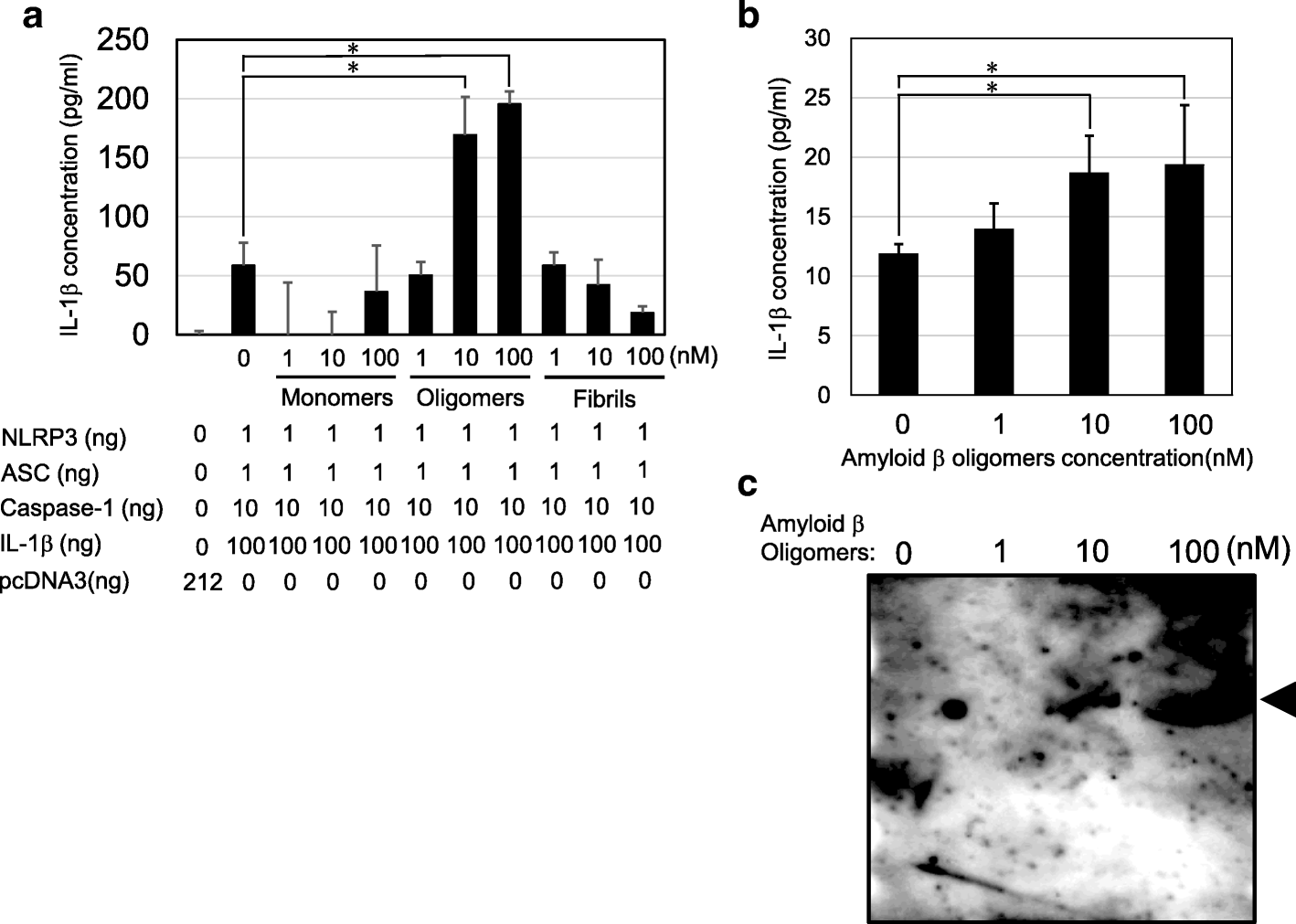
Amyloid β directly interacts with NLRP3 to initiate inflammasome

Cells, Free Full-Text
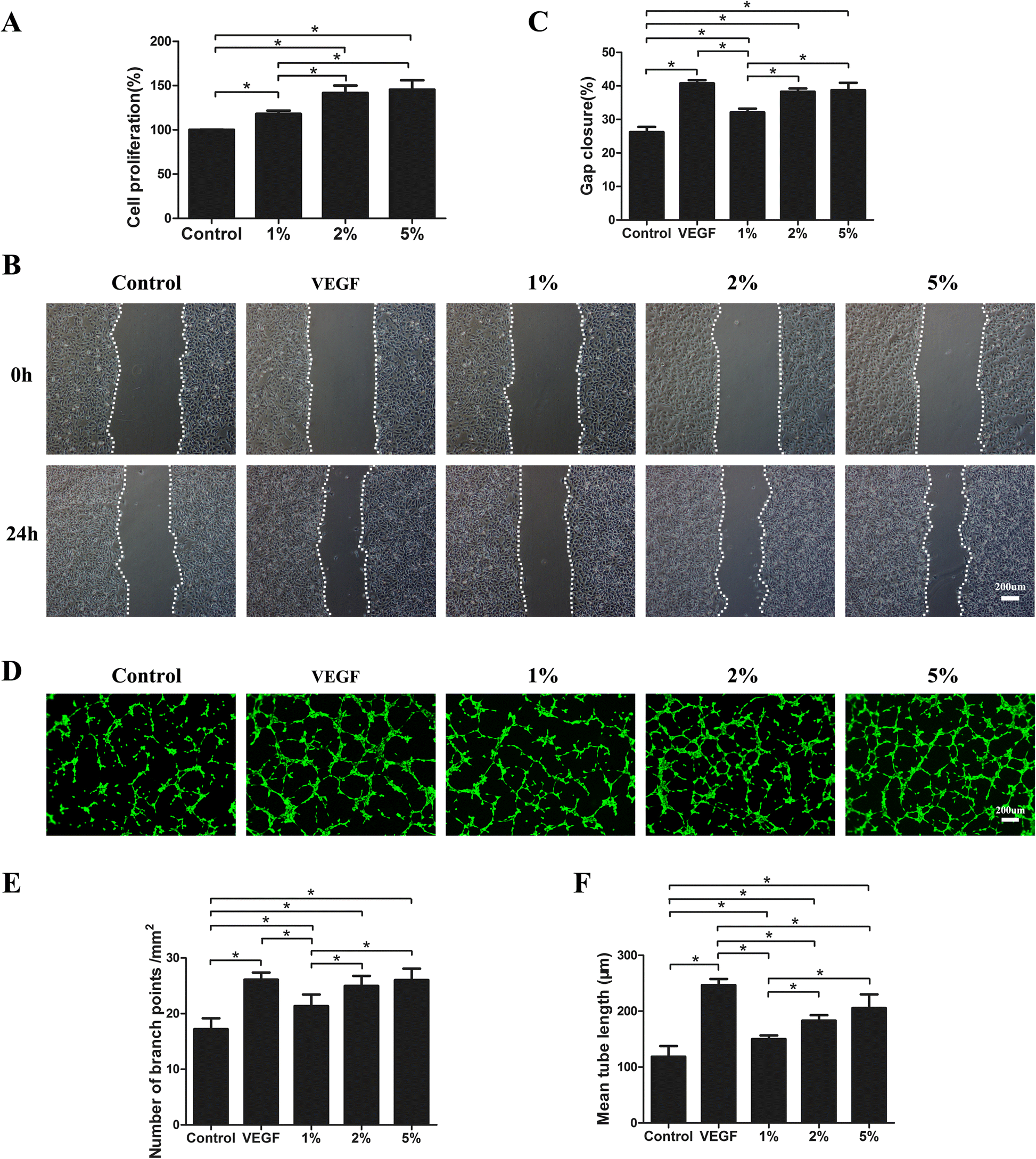
Fat extract promotes angiogenesis in a murine model of limb

Cell Circuits and Complex Tissues
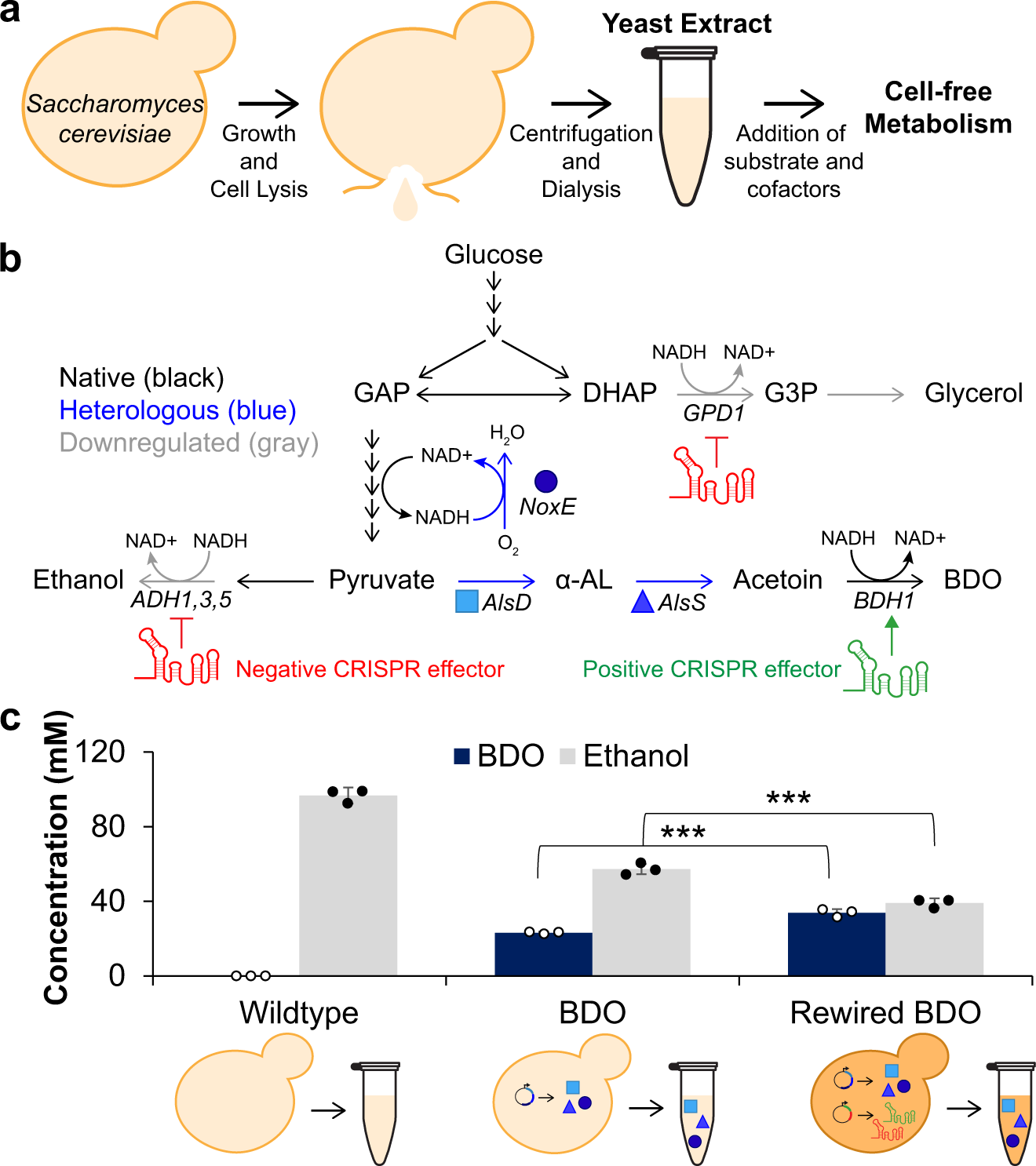
An integrated in vivo/in vitro framework to enhance cell-free

Cell-free DNA tissues of origin by methylation profiling reveals
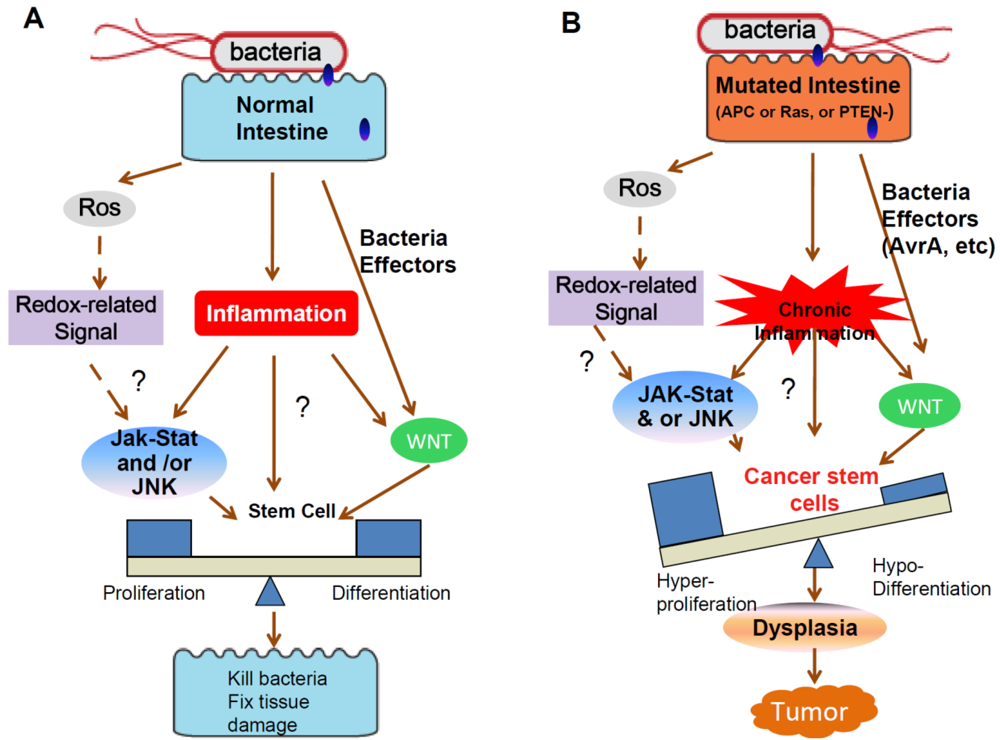
Cancers, Free Full-Text

Sequencing of Circulating Cell-free DNA during Pregnancy
de
por adulto (o preço varia de acordo com o tamanho do grupo)






:strip_icc()/i.s3.glbimg.com/v1/AUTH_b58693ed41d04a39826739159bf600a0/internal_photos/bs/2023/s/s/gwu349TAKX05b2fcvuYg/photo-2023-04-04-20-04-38.jpg)
