Life Sciences Commissioning, Qualification and Validation
Por um escritor misterioso
Descrição

Facilities and Utilities Commissioning & Qualification — Medvacon Life Sciences LLC

Release of 2nd Edition ISPE Baseline Guide 5 for C&Q - CAI
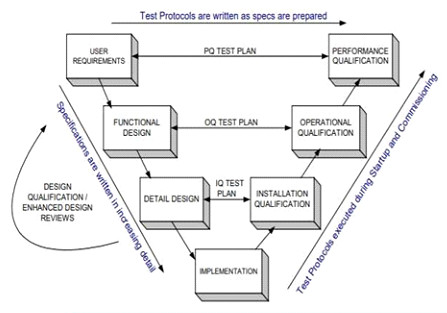
CQV

Commissioning, Qualification and Validation: A GMP Approach , Browne, Priscilla

Commissioning, Qualification, and Validation

VAL03: Commissioning and Installation Qualification
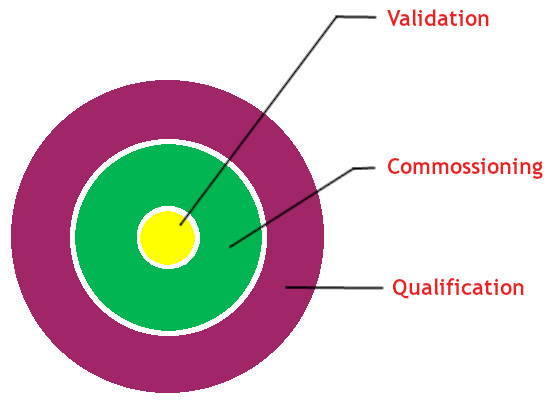
CQV

Effective Construction Program and Project Management

Commissioning, Qualification and Validation. - CSV Life Science
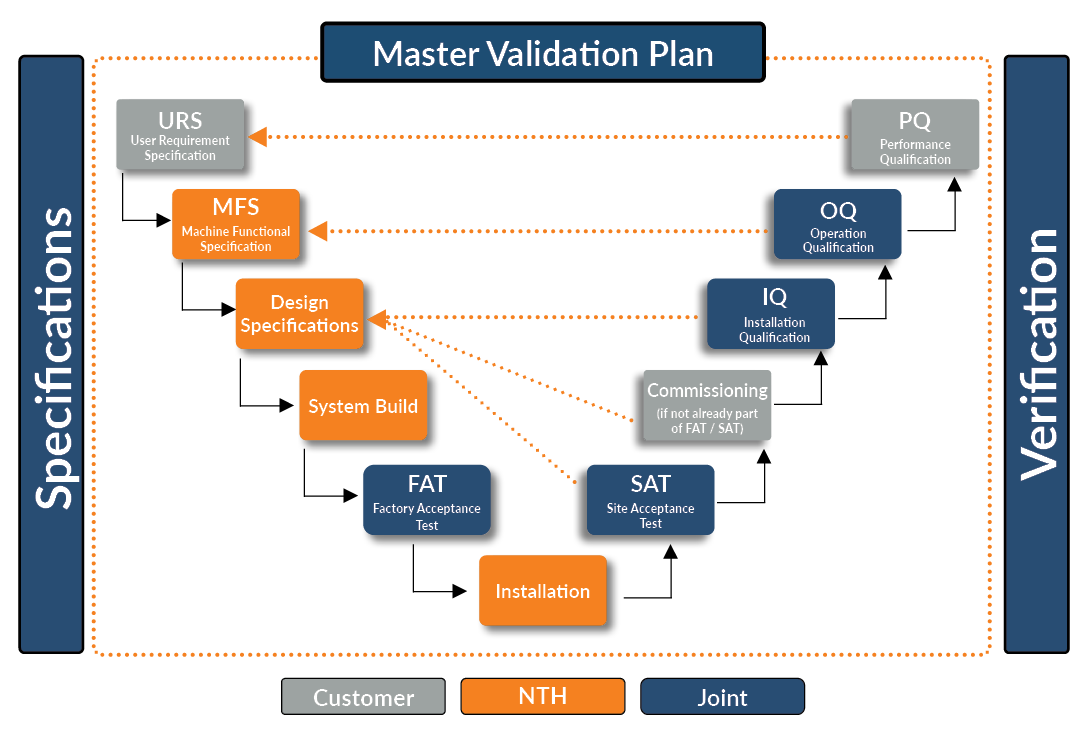
Simplifying GAMP Validation: Automation NTH Validation Support
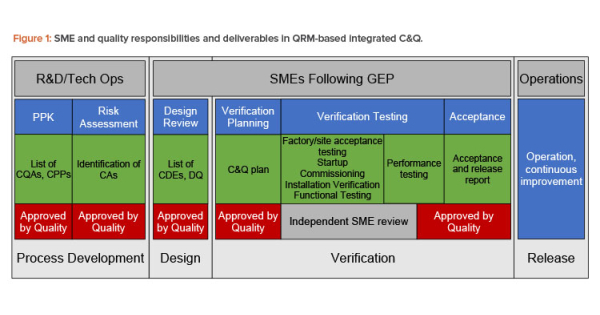
Good Engineering Practice in Risk-Based Commissioning & Qualification
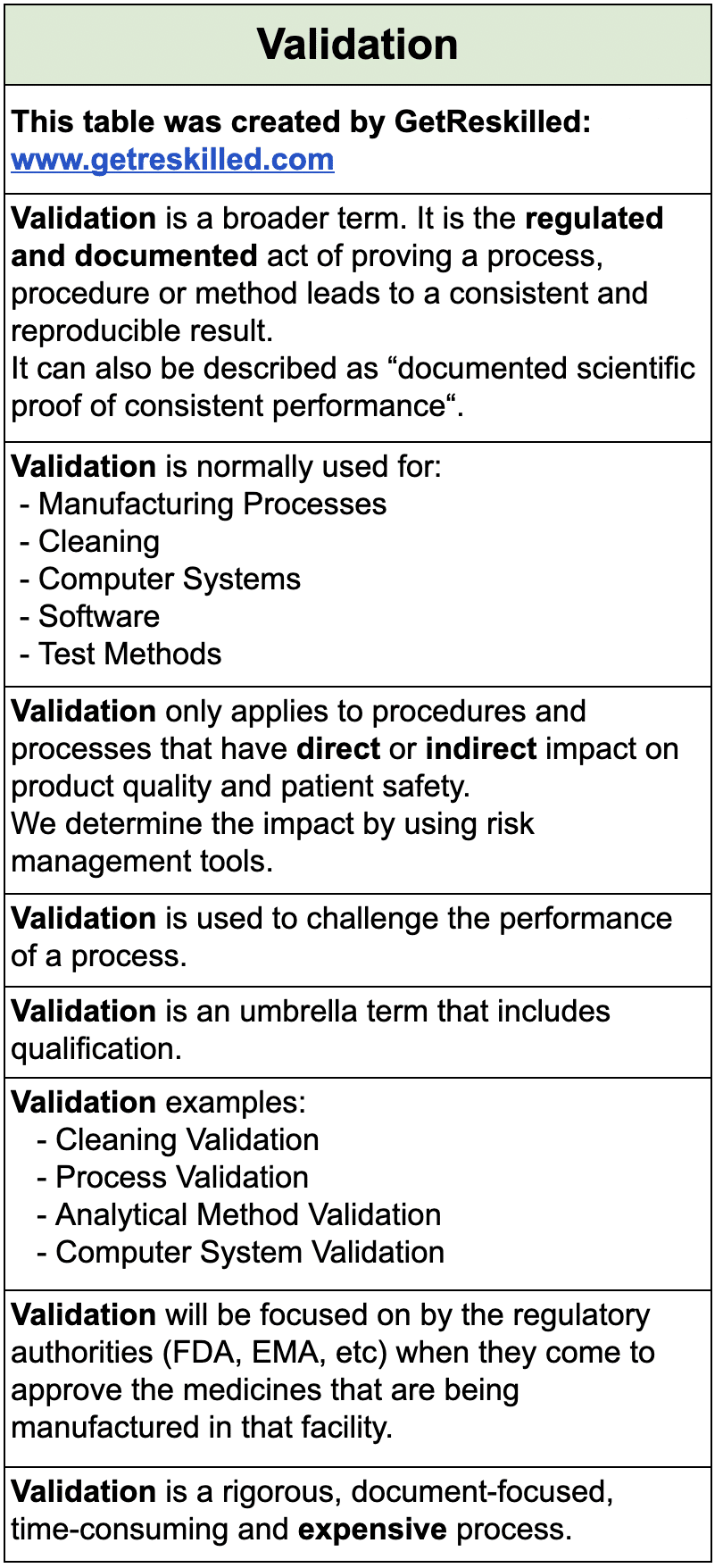
Commissioning vs Qualification vs Validation in Pharma
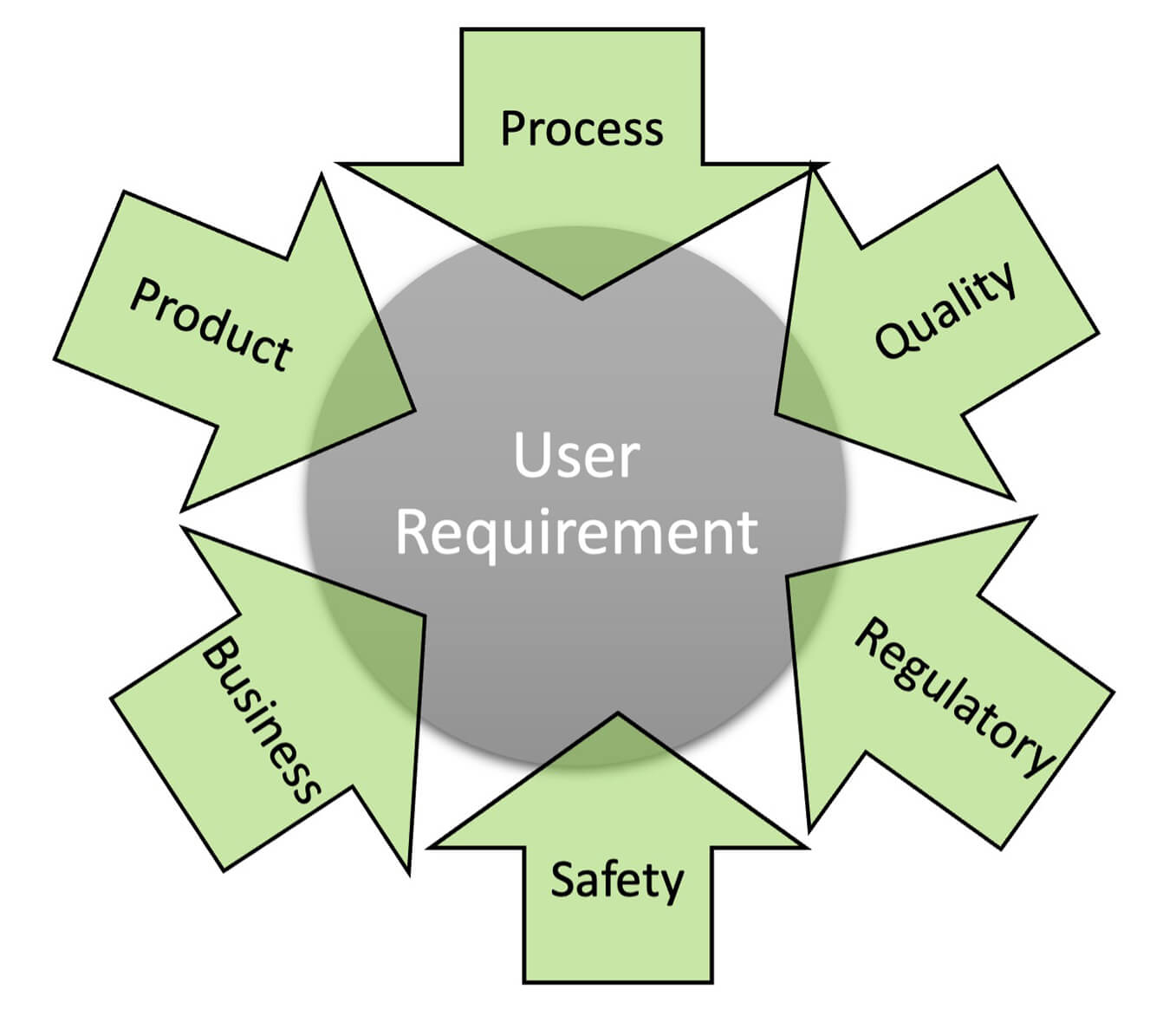
A Successful Q-Model for New mRNA Line Commissioning & Qualification: Pt. 1
Commissioning, Qualification and Validation (CQV) are requirements of modern facilities within the Life Science industry. Be it a Medical Device

Commissioning, Qualification and Validation: A GMP Approach
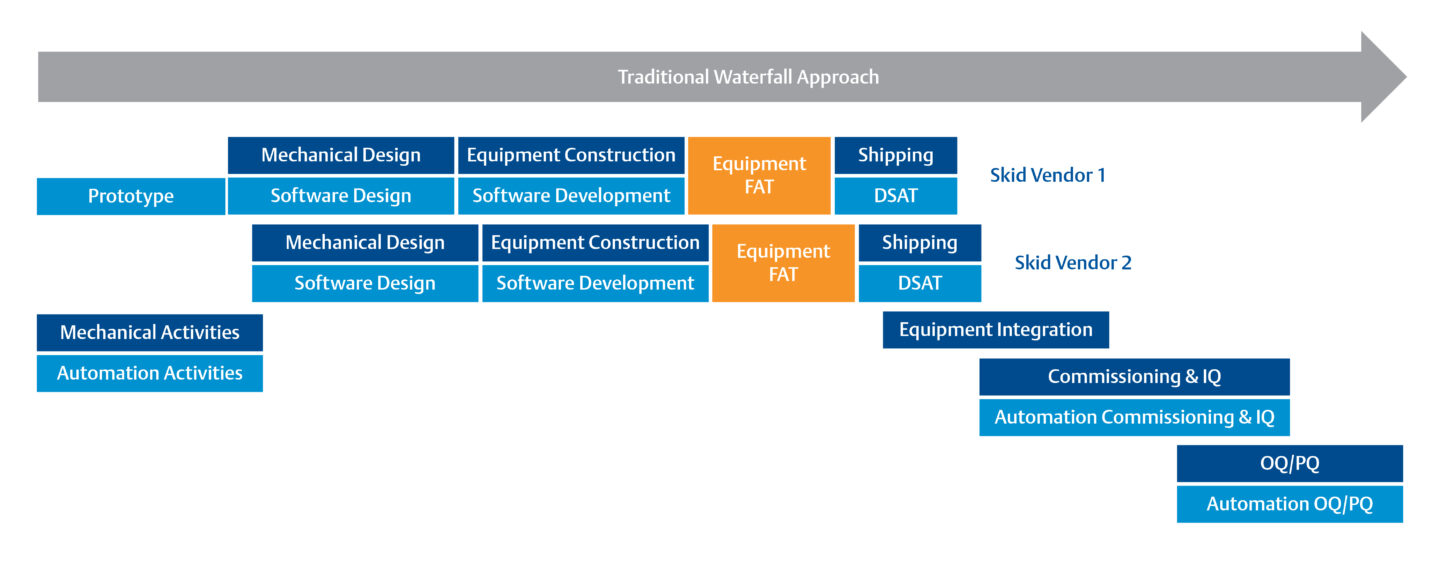
Shorten the Equipment Validation Process to Bring Projects Online Faster
de
por adulto (o preço varia de acordo com o tamanho do grupo)