ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)
Por um escritor misterioso
Descrição
ANANDA Scientific Inc., (a biotech pharma company) today announced approval by the U.S. Food and Drug Administration (FDA) of the Investigational New

Ananda Scientific (@AnandaScience) / X
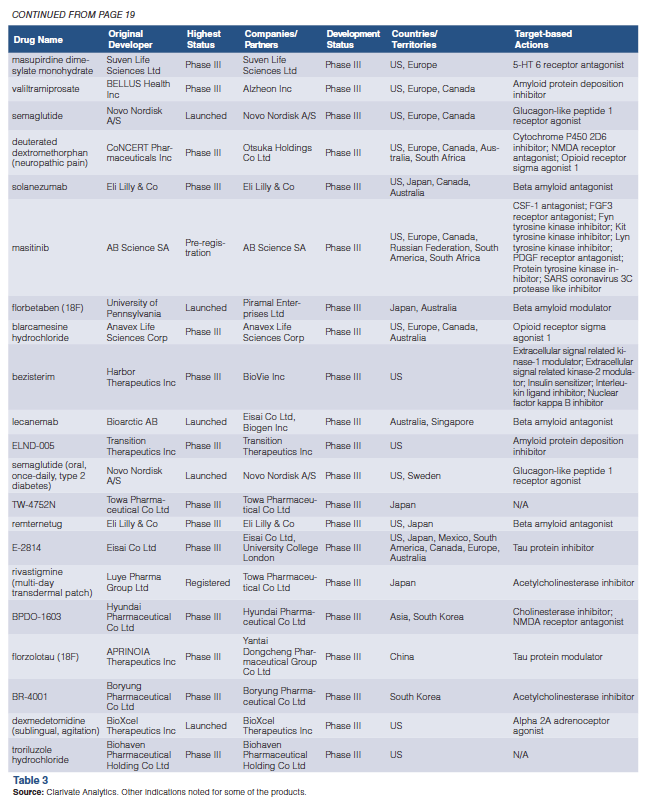
2024 Pipeline Report: First-World Focus

Does CBD Treatment Work For Opioid Addiction? - Addiction Resource

Ananda Scientific (@AnandaScience) / X

Blog Articles And Insights

Nantheia ATL5: A New Hope for Beating Opioid Addiction - Treatment Magazine

Use of Medication-Assisted Treatment for Opioid Use Disorders in Employer-Sponsored Health Insurance: Final Report

Media – Ananda Scientific

Justin Molignoni CRNP on LinkedIn: ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial…

Can CBD Help Get People Off Opioids? - Biotech Firms Gets FDA Approval to Study CBD-Based Drug for Opioid Addiction
de
por adulto (o preço varia de acordo com o tamanho do grupo)







