New approach methodologies in human regulatory toxicology – Not if, but how and when! - ScienceDirect
Por um escritor misterioso
Descrição

Incorporating lines of evidence from New Approach Methodologies (NAMs) to reduce uncertainties in a category based read-across: A case study for repeated dose toxicity - ScienceDirect

Testing the feasibility of a new way of toxicity testing and reduction
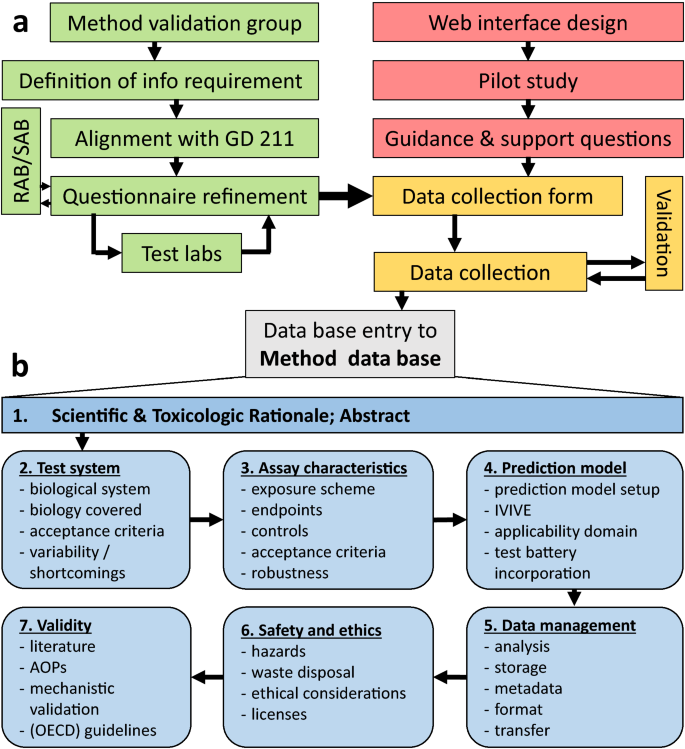
The EU-ToxRisk method documentation, data processing and chemical testing pipeline for the regulatory use of new approach methods

Acceptable Intakes (AIs) for 11 Small molecule N-nitrosamines (NAs) -Pub - New Scientific Knowledge & Development - Nitrosamines Exchange

Thomas Petry, Ph.D. on LinkedIn: Is the EU chemicals strategy for sustainability a green deal? To be…

Regulatory assessment of chemical mixtures: Requirements, current approaches and future perspectives - ScienceDirect

Paving the way for application of next generation risk assessment to safety decision-making for cosmetic ingredients - ScienceDirect

Regulatory risk assessment approaches for synthetic mineral fibres – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free on CyberLeninka open science hub.

Dietary predictors of urinary biomarkers of pyrethroids in the general population- A scoping review - The Journal of Nutrition

New Approach Methodologies (NAMs) for safety testing of complex food matrices: A review of status, considerations, and regulatory adoption - ScienceDirect

Pharmaceutical toxicology: Designing studies to reduce animal use, while maximizing human translation - ScienceDirect
de
por adulto (o preço varia de acordo com o tamanho do grupo)







