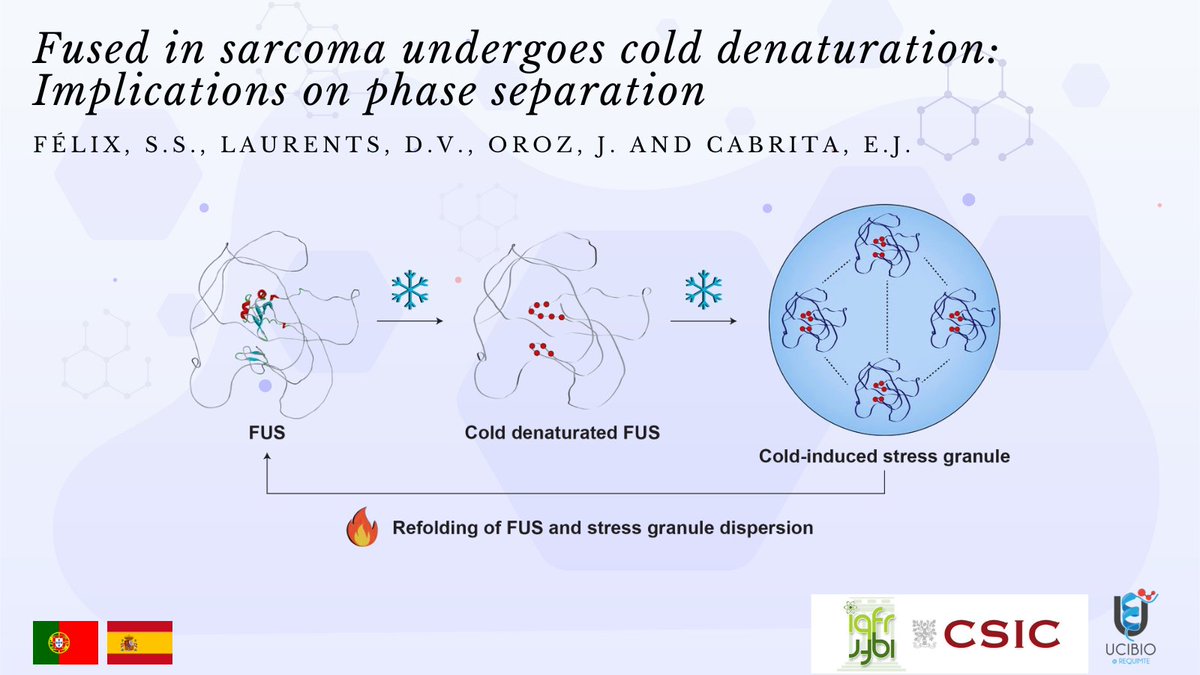
Fused in sarcoma undergoes cold denaturation: Implications on phase separation
Por um escritor misterioso
Descrição
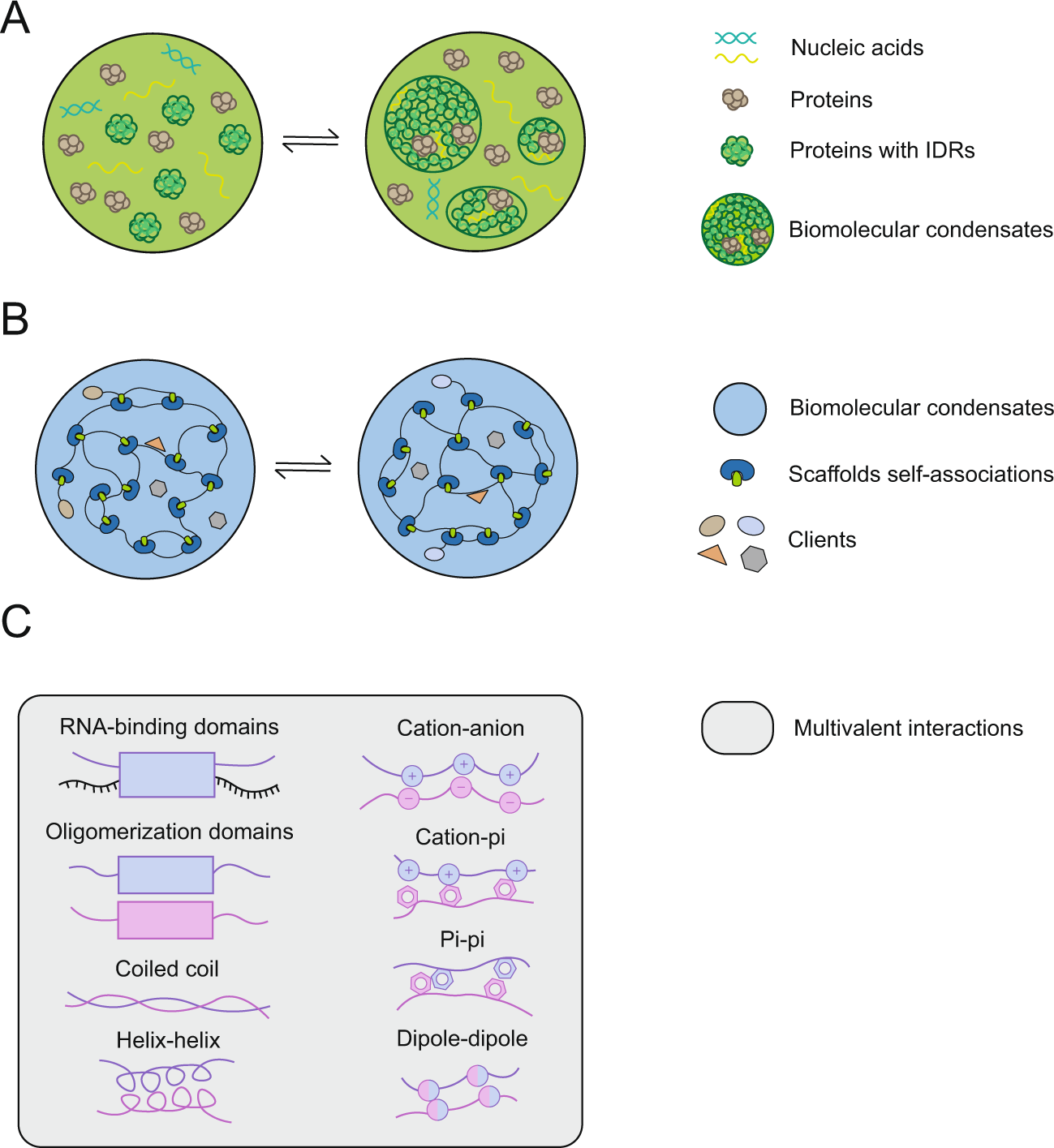
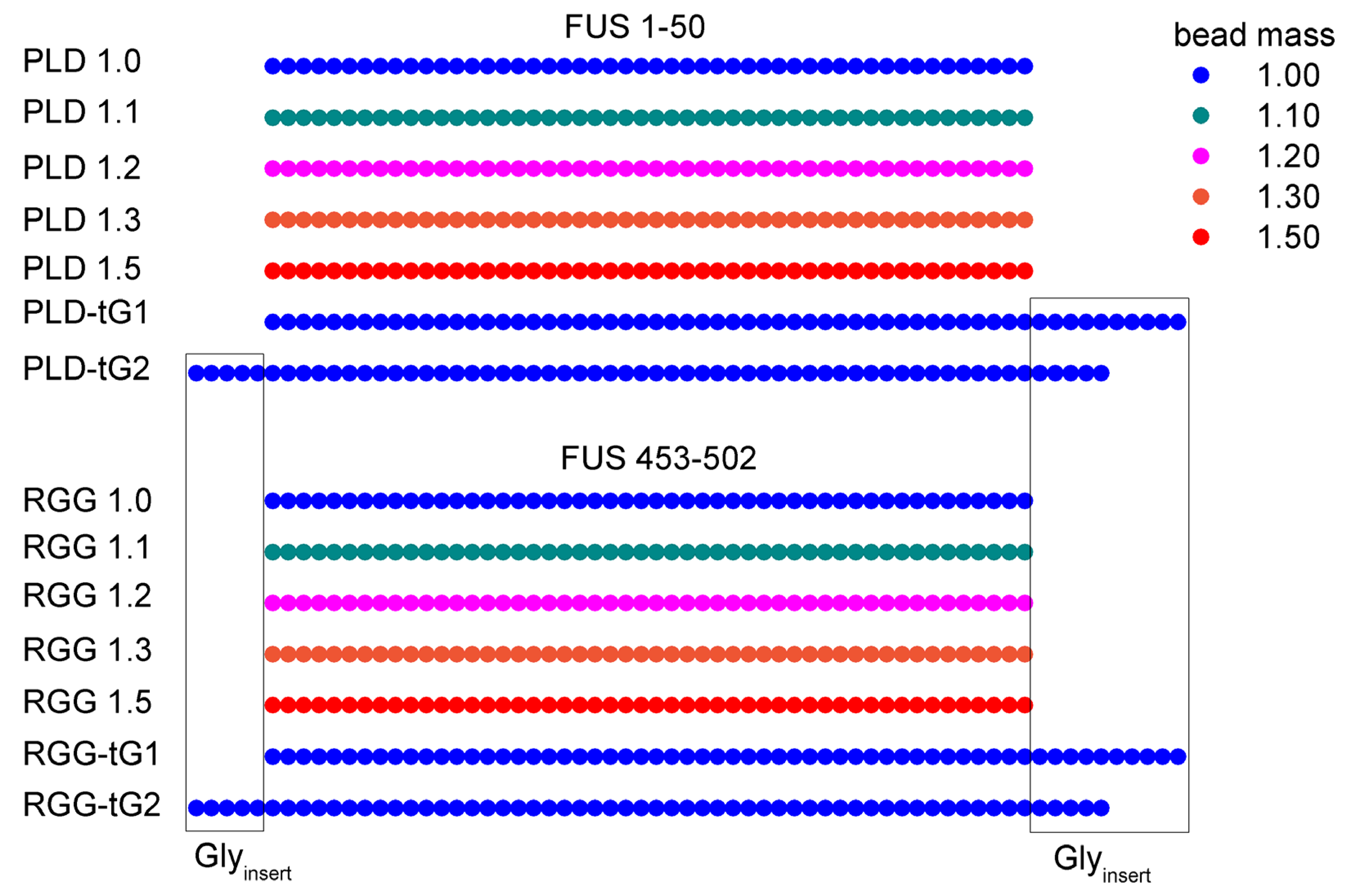
The mediation of fused in sarcoma (FUS) protein liquid-liquid phase separation (LLPS) is generally attributed to the low-complexity and disordered domains, while the role of its folded domains remains unknown. In this work we questioned the role of the folded domains on the full-length (FL) FUS LLPS and studied the influence of several metabolites, ions and overall conditions on the LLPS process using turbidity assays, differential interference contrast microscopy and nuclear magnetic resonance spectroscopy. We demonstrate that FL FUS LLPS is highly responsive to the surrounding conditions, and that overall intrinsic disorder is crucial for LLPS. To promote such disorder, we reveal that the FUS RNA-recognition domain (RRM) and the zinc-finger motif (ZnF) undergo cold denaturation above 0ºC, at a temperature that is determined by the conformational stability of the ZnF domain. We hypothesize that, in cold shock conditions, cold denaturation might provide a pathway that exposes additional residues to promote FUS self-assembly. Such findings mark the first evidence that FUS globular domains may have an active role in stress granule formation in cold stress.

Liquid–Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation - ScienceDirect
Sofia Ferreira (@Figueirinhalq) / X

FUS is recruited to sites of DNA damage and contributes to DNA-damage

Liquid–Liquid Phase Separation? Ask the Water!

Liquid–Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation - ScienceDirect
Phase separation drives tumor pathogenesis and evolution: all roads lead to Rome

Cold Denaturation of the HIV-1 Protease Monomer

Effect of the number of pipetting strokes on the FUS fluorescence

Intrinsically disordered protein's coil-to-globule transition and adsorption onto a hydrophobic surface under different conditions
Biomolecules, Free Full-Text
Figure S3. Comparison of the 15 N HSQC NMR spectra of wild type Yfh1

Dynamic similarity scaling of USAXS data by I max and q max , for the

PDF] Fused in Sarcoma: Properties, Self-Assembly and Correlation with Neurodegenerative Diseases
de
por adulto (o preço varia de acordo com o tamanho do grupo)