Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial - The Lancet
Por um escritor misterioso
Descrição

News at XI: moving beyond factor Xa inhibitors - ScienceDirect

7. Inhibición del Factor XIa con asundexian tras ictus isquémico

Anticoagulation
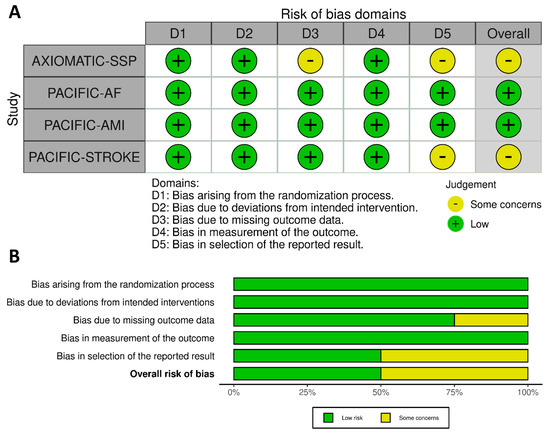
JCM, Free Full-Text

Determination of the Potential Clinical Benefits of Small Molecule
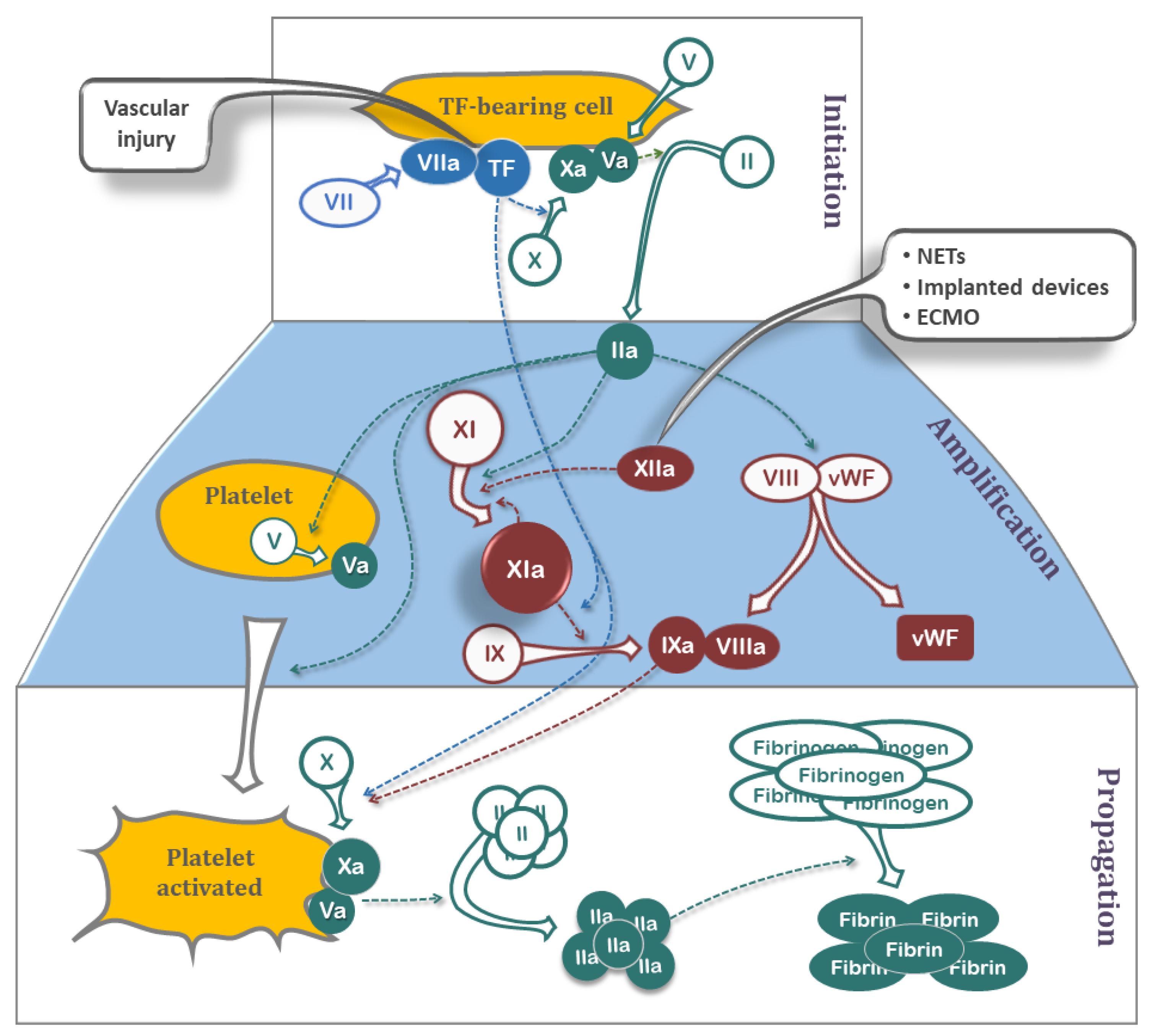
JCDD, Free Full-Text

Full article: New pharmacotherapeutic options for oral

Antithrombotic Therapy for Primary and Secondary Prevention of

Determination of the Potential Clinical Benefits of Small Molecule

Factor XIa inhibition with asundexian after acute non

Frequency and Patterns of Brain Infarction in Patients With

Antithrombotic Therapy for Primary and Secondary Prevention of

Safety of the oral factor XIa inhibitor asundexian compared with
de
por adulto (o preço varia de acordo com o tamanho do grupo)